PRMT5
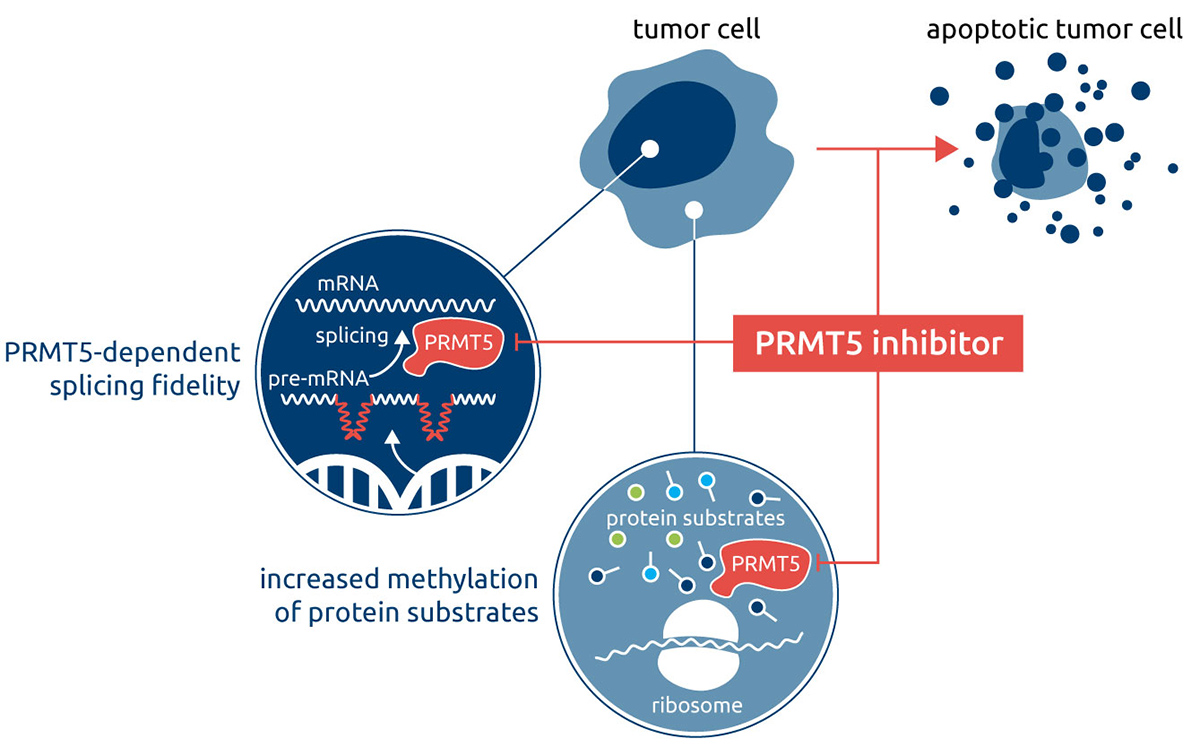
The methyltransferase PRMT5 (protein arginine methyl transferase 5) catalyzes the transfer of a methyl group from the co-factor S-adenosylmethione (SAM) to arginine residues in a target protein, thereby regulating the activity of a range of protein substrates, many of which are involved in key cellular processes. PRMT5 is upregulated in a variety of human malignancies. In cancer cell lines, increased PRMT5 expression is correlated with growth rate while in patient-derived primary tumors it is correlated with poor patient survival.
In cancer, the main mechanism of action of PRMT5 inhibition is to modulate alternative RNA splicing. Many hematological and solid tumors are known to hijack the cell’s splicing machinery to maintain splicing fidelity and drive tumorigenesis and tumor growth. This renders PRMT5 – a key splicing regulator – an exploitable vulnerability in such tumors. Indeed, in pre-clinical models, pharmacological inhibition of PRMT5 induces robust anti-tumor activity, further highlighting the emergence of PRMT5 as a promising cancer drug target.
Lead Pharma has both SAM cooperative and MTA cooperative series in development.

